Tetrahydrocannabinol, abbreviated THC, is one of at least 113 cannabinoids identified in cannabis. THC is the principal psychoactive constituent of cannabis. With chemical name, (-)-trans-??-tetrahydrocannabinol, the term THC also refers to cannabinoid isomers. Dronabinol is a synthetic form of THC approved by the FDA as an appetite stimulant for people with AIDS and antiemetic for people receiving chemotherapy. The pharmaceutical formulation dronabinol is an oily resin provided in capsules available by prescription in the United States, Canada, Germany, and New Zealand.
Like most pharmacologically-active secondary metabolites of plants, THC is a lipid found in cannabis, assumed to be involved in the plant's self-defense, putatively against insect predation, ultraviolet light, and environmental stress.
THC, along with its double bond isomers and their stereoisomers, is one of only three cannabinoids scheduled by the UN Convention on Psychotropic Substances (the other two are dimethylheptylpyran and parahexyl). It was listed under Schedule I in 1971, but reclassified to Schedule II in 1991 following a recommendation from the WHO. Based on subsequent studies, the WHO has recommended the reclassification to the less-stringent Schedule III. Cannabis as a plant is scheduled by the Single Convention on Narcotic Drugs (Schedule I and IV). It is specifically still listed under Schedule I by US federal law under the Controlled Substances Act passed by the US Congress in 1970.
Video Tetrahydrocannabinol
Medical uses
THC is an active ingredient in nabiximols, a specific extract of Cannabis that was approved as a botanical drug in the United Kingdom in 2010 as a mouth spray for people with multiple sclerosis to alleviate neuropathic pain, spasticity, overactive bladder, and other symptoms.
Maps Tetrahydrocannabinol
Pharmacology
Mechanism of action
The actions of THC result from its partial agonist activity at the cannabinoid receptor CB1 (Ki=10nM), located mainly in the central nervous system, and the CB2 receptor (Ki=24nM), mainly expressed in cells of the immune system. The psychoactive effects of THC are primarily mediated by the activation of cannabinoid receptors, which result in a decrease in the concentration of the second messenger molecule cAMP through inhibition of adenylate cyclase.
The presence of these specialized cannabinoid receptors in the brain led researchers to the discovery of endocannabinoids, such as anandamide and 2-arachidonoyl glyceride (2-AG). THC targets receptors in a manner far less selective than endocannabinoid molecules released during retrograde signaling, as the drug has a relatively low cannabinoid receptor efficacy and affinity. In populations of low cannabinoid receptor density, THC may act to antagonize endogenous agonists that possess greater receptor efficacy. THC is a lipophilic molecule and may bind non-specifically to a variety of entities in the brain and body, such as adipose tissue (fat).
THC, similarly to cannabidiol, albeit less potently, is a positive allosteric modulator of the ?- and ?-opioid receptors.
Due to its partial agonistic activity, THC appears to result in greater downregulation of cannabinoid receptors than endocannabinoids, further limiting its efficacy over other cannabinoids. While tolerance may limit the maximal effects of certain drugs, evidence suggests that tolerance develops irregularly for different effects with greater resistance for primary over side-effects, and may actually serve to enhance the drug's therapeutic window. However, this form of tolerance appears to be irregular throughout mouse brain areas. THC, as well as other cannabinoids that contain a phenol group, possesses mild antioxidant activity sufficient to protect neurons against oxidative stress, such as that produced by glutamate-induced excitotoxicity.
Pharmacokinetics
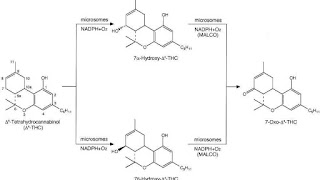
THC is metabolized mainly to 11-OH-THC by the body. This metabolite is still psychoactive and is further oxidized to 11-nor-9-carboxy-THC (THC-COOH). In humans and animals, more than 100 metabolites could be identified, but 11-OH-THC and THC-COOH are the dominating metabolites. Metabolism occurs mainly in the liver by cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP3A4. More than 55% of THC is excreted in the feces and ~20% in the urine. The main metabolite in urine is the ester of glucuronic acid and THC-COOH and free THC-COOH. In the feces, mainly 11-OH-THC was detected.
Physical and chemical properties
Discovery and structure identification
In 1940, cannabidiol was isolated and identified from Cannabis sativa, and THC was isolated and described for its structure and synthesis in 1964.
Solubility
As with many aromatic terpenoids, THC has a very low solubility in water, but good solubility in most organic solvents, specifically lipids and alcohols.
Total Synthesis
A total synthesis of the compound was reported in 1965; that procedure called for the intramolecular alkyl lithium attack on a starting carbonyl to form the fused rings, and a tosyl chloride mediated formation of the ether.
Biosynthesis
In the Cannabis plant, THC occurs mainly as tetrahydrocannabinolic acid (THCA, 2-COOH-THC, THC-COOH). Geranyl pyrophosphate and olivetolic acid react, catalysed by an enzyme to produce cannabigerolic acid, which is cyclized by the enzyme THC acid synthase to give THCA. Over time, or when heated, THCA is decarboxylated, producing THC. The pathway for THCA biosynthesis is similar to that which produces the bitter acid humulone in hops.
Detection in body fluids
THC and its 11-OH-THC and THC-COOH metabolites can be detected and quantified in blood, urine, hair, oral fluid or sweat using a combination of immunoassay and chromatographic techniques as part of a drug use testing program or in a forensic investigation.
History
THC was first isolated in 1964 by Raphael Mechoulam and Yechiel Gaoni at the Weizmann Institute of Science in Israel.
At its 33rd meeting, in 2003, the World Health Organization Expert Committee on Drug Dependence recommended transferring THC to Schedule IV of the Convention, citing its medical uses and low abuse potential.
Society and culture
Comparisons with medical cannabis
Female cannabis plants contain at least 113 cannabinoids, including cannabidiol (CBD), thought to be the major anticonvulsant that helps people with multiple sclerosis; and cannabichromene (CBC), an anti-inflammatory which may contribute to the pain-killing effect of cannabis.
Research
The status of THC as an illegal drug in most countries imposes restrictions on research material supply and funding, such as in the United States where the National Institute on Drug Abuse and Drug Enforcement Agency regulated sources of cannabis for researchers until August 2016 when licenses were provided to growers for supplies of medical marijuana. Although cannabis is legalized for medical uses in half of the United States, no products have been approved for federal commerce by the Food and Drug Administration, a status that limits cultivation, manufacture, distribution, clinical research, and therapeutic applications.
In April 2014, the American Academy of Neurology found evidence supporting the effectiveness of the cannabis extracts in treating certain symptoms of multiple sclerosis and pain, but there was insufficient evidence to determine effectiveness for treating several other neurological diseases. A 2015 review confirmed that medical marijuana was effective for treating spasticity and chronic pain, but caused numerous short-lasting adverse events, such as euphoria and dizziness.
Multiple sclerosis symptoms
- Spasticity. Based on the results of 3 high quality trials and 5 of lower quality, oral cannabis extract was rated as effective, and THC as probably effective, for improving people's subjective experience of spasticity. Oral cannabis extract and THC both were rated as possibly effective for improving objective measures of spasticity.
- Centrally mediated pain and painful spasms. Based on the results of 4 high quality trials and 4 low quality trials, oral cannabis extract was rated as effective, and THC as probably effective in treating central pain and painful spasms.
- Bladder dysfunction. Based on a single high quality study, oral cannabis extract and THC were rated as probably ineffective for controlling bladder complaints in multiple sclerosis
Neurodegenerative disorders
- Huntington disease. No reliable conclusions could be drawn regarding the effectiveness of THC or oral cannabis extract in treating the symptoms of Huntington disease as the available trials were too small to reliably detect any difference
- Parkinson's disease. Based on a single study, oral CBD extract was rated probably ineffective in treating levodopa-induced dyskinesia in Parkinson's disease.
- Alzheimer's disease. A 2011 Cochrane Review found insufficient evidence to conclude whether cannabis products have any utility in the treatment of Alzheimer's disease.
Other neurological disorders
- Tourette syndrome. The available data was determined to be insufficient to allow reliable conclusions to be drawn regarding the effectiveness of oral cannabis extract or THC in controlling tics.
- Cervical dystonia. Insufficient data was available to assess the effectiveness of oral cannabis extract of THC in treating cervical dystonia.
- Epilepsy. Data was considered insufficient to judge the utility of cannabis products in reducing seizure frequency or severity.
See also
- Cannabinoids
- 11-Hydroxy-THC, metabolite of THC
- Anandamide, 2-Arachidonoylglycerol, endogenous cannabinoid agonists
- Cannabidiol (CBD), an isomer of THC
- Cannabinol (CBN), a metabolite of THC
- Dimethylheptylpyran
- Parahexyl
- Tetrahydrocannabinolic acid, the biosynthetic precursor for THC
- HU-210, WIN 55,212-2, JWH-133, synthetic cannabinoid agonists
- Medical cannabis
- Nabilone
- War on Drugs
- Effects of cannabis
- Cannabinoid hyperemesis syndrome
- List of investigational analgesics
References
External links
- U.S. National Library of Medicine: Drug Information Portal - Tetrahydrocannabinol
Source of article : Wikipedia